Develop, manufacture and deliver affordable treatment to millions of patients.
Integrated model
We are a leading producer and distributor of health related products. Our business model is vertically integrated running through the whole value chain.
 Main Pharmaceutical Manufacturing Companies Sopharma AD Unipharm AD Biopharm Engineering AD
Main Non-Pharmaceutical Manufacturing Companies Medica AD Momina Krepost AD Biopharm Engineering AD |
15 |
traditional herbal productsin the mother company |
| more than 200 |
generic productsin the Group |
|
| 5 |
of top 10 most sold productsof the mother company |
 Main Distribution Companies Sopharma Trading AD BRIZ Latvia |
Sopharma Trading is a Bulgarian Market leader in the wholesale sector |
|
Partner of choice |
||
400 partner and
|
Sustainable Development
Our cost structure coupled with the know-how and GMP certifications of our production facilities enable us to offer high quality competitively priced products. We aim at providing more accessible and affordable treatment than our competitors.
We have a strong potential for further development on Sopharma’s own traditional herbal products in the long-term. The first such product registered on the Austrian market was Nivaject, the injectable form of Nivalin (galantamin).
Product cycle
-
Health Issues
Sopharma AD is focused on identifying socially important diseases and on developing, and presenting to the market affordable medicines to control them. Thus the company contributes to people` improved health and prolonged life.
-
R&D
This stage of the product cycle includes the development of molecules that solves the particular health issues identified in step one.
-
Clinical Trials
To evaluate its medicinal products, Sopharma AD conducts clinical trials. New generic products are clinically tested for bioequivalence. Sopharma AD also takes responsibility to gather additional information about a medicine's safety, efficacy and optimal use after the regulatory approval. For its branded products the company performs post-authorization studies.
-
Registration
Once the pharmaceutical and clinical development is accomplished, the dossier of the product is prepared to serve the purposes of Marketing Authorization Application. Following a procedure of Regulatory Authority review and approval the product is granted a Marketing Authorization on certain territory/ies.
-
Production
The production process is in full compliance with GMP regulations and makes.
-
Quality Management
Quality assurance and control is present in all stepst of the product cycle, including, but not limited to the production process. To make sure that a patient receives always the same product different measures and control steps are taken at each stage to provide feedback on the main parameters of the product.
-
Marketing
the marketing effort is designed to achieve one main purpose – to provide information for the availability of a new and efficient treatment to the medical professionals and patients.
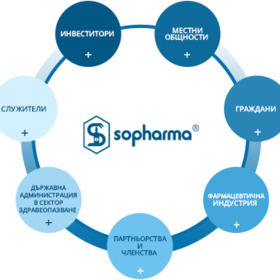
Our social role: innovative approach to the health care experience and affordable treatment
A socially engaged domestic player with leading market position
The Group is present in 33 countries, but the key markets are Bulgaria, Russia, Ukraine, Kazakhstan and Poland and the strategic plan focuses on further market expansion within the area.
Manufacturing Facilities

Production facilities:
Sofia
Vrabevo
Kazanlak
Sliven
Veliko Tarnovo
Sandanski
Distribution Network
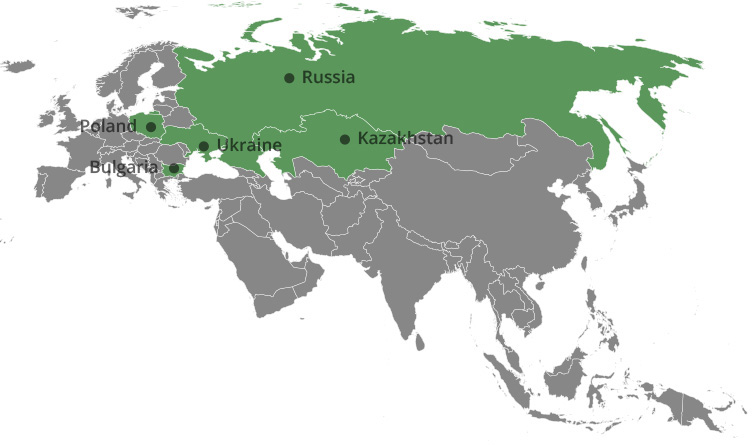
Distribution network:
Bulgaria, Russia, Ukraine, Kazakhstan, Poland
Bulgaria: Sopharma Trading AD – a business entity registered in Bulgaria with a seat and address of management: Sofia, Izgrev Region, 5 Lachezar Stanchev Str., Sopharma Business Towers, Building A, floor 12. Sopharma Trading AD is a pharmaceutical distribution company providing wholesale and pre-wholesale services to pharmaceutical and healthcare related producers. The company is number one on the hospital market and has a leading position on the retail market in Bulgaria with more than 20% market share.
Ukraine: Sopharma Ukraine is the distribution company responsible for Sopharma products on the Ukrainian market. The company is registered in the Ukraine with a seat and address of management: Ukraine, Kiev, Oblonski Region, prospect Moskovskii No. 9, unit 4, floor 2, office 4-203.
Kazakhstan: TOO Sopharma Kazakhstan, Kazakhstan – a business entity registered in Kazakhstan with a seat and registered address: Kazakhstan, Almaty, Auezovski Region, Mamyr Microdistrict - 4, d. 190 responsible for distributing Sopharma’s products in Kazakhstan.
Poland: Sopharma Warsaw SP. Z.O.O., Poland – a business entity registered in Poland with a seat and address of management: Poland, Warsaw, 8, Halubinskiego Str. Responsible for distribution of Sopharma’s products in Poland.
Latvia, Litva and Belarus: Briz SIA, Latvia – a business entity registered in Latvia with a seat and address of management: Latvia, Riga, Rasas No. 5, LV – 1057.
We are
-
Innovation enabler
Continuosly investing in technologies, innovation, research and science
-
Health distributor
Increasingly enhancing a distribution model for products and services that make medication more affordable and accesible for the vast majority of patients
-
Reliable partner
Establishing a constant and effective dialogue with all parties in the pharmaceutical industry that contribute to the improvement of the health care system
-
A valuable investment
Thanks to
- a business strategy focused on portfolio diversification, constant expansion on foreign markets, successful M&A operations
- a financial strategy based on profitability and on strong performance in key markets
- adoption of transparent and efficient governance model